ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved in one or more stages of the  life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities. ISO 13485:2016 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organizations.
life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities. ISO 13485:2016 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organizations.
Requirements of ISO 13485:2016 are applicable to organizations regardless of their size and regardless of their type except where explicitly stated. Wherever requirements are specified as applying to medical devices, the requirements apply equally to associated services as supplied by the organization.”
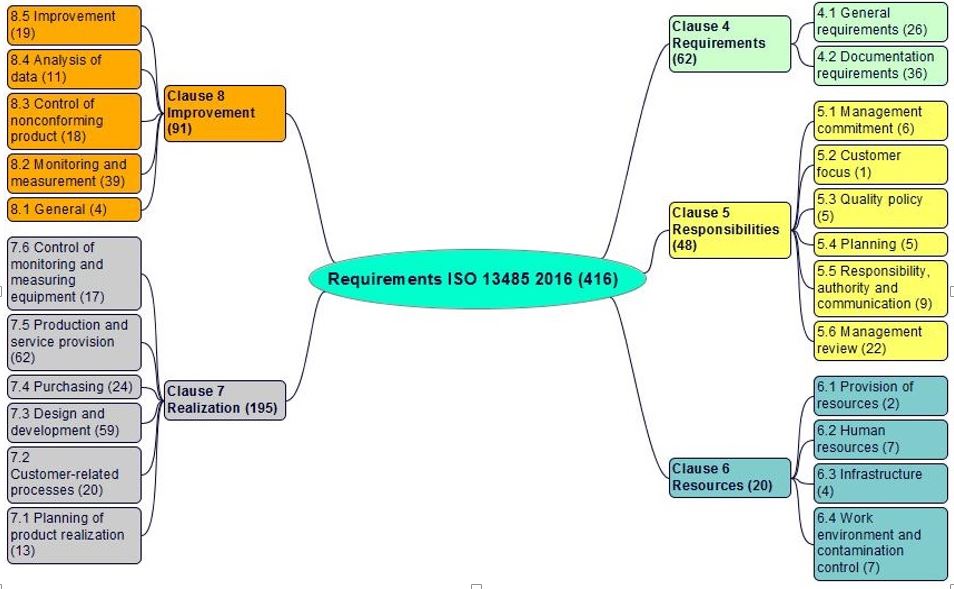
ISO 13485:2016 Clauses:
 Implementation
Implementation
There are a number of steps that are necessary to implement a Medical Devices Quality Management System
1. Top Management Commitment
The most important step in implementing a Medical Devices Quality Management System that will meet or exceed an ISO 13485 standard is to acquire the full support of upper management
2. Appoint the Management Representative
Once the commitment has been made, the process can proceed by adopting a project team approach and treating it the same as any other business undertaking. The next step is the appointment of a Management Representative.
3. Awareness
This step acquires an awareness program.
4. Appoint an Implementation team
After everyone has been informed about the organisation’s intentions to develop the Medical Devices Quality Management System, an implementation team should be assembled.
5. Training
The implementation team, supervisors, and internal audit team should be trained.
6. Time Schedule
This activity develops a time schedule for the implementation and registration of the system.
7. Select Element Owners
The implementation team selects owners for each of the system elements.
8. Review the Present System
Perform a review of the present Medical Devices Quality Management System.
9. Write the Documents
Prepare written Medical Devices Quality policy and procedure manuals. Write appropriate work instructions to maintain the Medical Devices Quality Management System.
10. Install the New System
Integrate the policies, procedures, and work instructions into day-to-day workings of the organisation.
11. Internal Audit
Conduct an internal audit of the Medical Devices Quality management System.
12. Management Review
Conduct a management review.
13. Pre-assessment
This step is optional. If a good job has been done on the previous steps, pre-assessment is not necessary.
14. Registration
This step has three parts: Choosing a certification body, submitting an application, and conducting the certification body’s system audit.

 Implementation
Implementation